 "ddavidn" (ddavidn)
"ddavidn" (ddavidn)
08/28/2013 at 14:52 • Filed to: Edumacation
 0
0
 21
21
 "ddavidn" (ddavidn)
"ddavidn" (ddavidn)
08/28/2013 at 14:52 • Filed to: Edumacation |  0 0
|  21 21 |
...And it got me wondering about the different types of antifreeze, which got me wondering exactly what it does/how it works. What would happen if I used a whole jug of full strength instead of 50/50? What happens if I use the wrong type? I used the Googlenet, but I wasn't sure who I could trust. So, of course, I trust Oppo completely. Point me in the right direction so that I have something to read at lunchtime after I watch /DRIVE CLEAN. Please and thanks.
 desertdog5051
> ddavidn
desertdog5051
> ddavidn
08/28/2013 at 14:58 |
|
I remember reading something on that a few years ago. Don't recall exactly but you are not supposed to run straight antifreeze.
 ddavidn
> desertdog5051
ddavidn
> desertdog5051
08/28/2013 at 14:58 |
|
Don't worry, it wasn't a mistake I made. I was just wondering as I poured. I wouldn't want to waste money by pouring 1.5gal of concentrate anyway.
 RamblinRover Luxury-Yacht
> ddavidn
RamblinRover Luxury-Yacht
> ddavidn
08/28/2013 at 15:00 |
|
> 50/50: higher boiling point, lower freezing point, BUT - IIRC lower thermal capacity. 50/50 is standard easy thing for the end user to prepare, makes for reasonable shipping, and typically answers to the best compromise of heating, flow properties, and freeze/boil resistance.
Using the wrong type, due to silicates and other compounds present, can cause precipitates to form out of the mix of two types and clog things. In addition to this, some types of antifreeze don't play well with this or that metal or compound in a cooling system.
Basic description of antifreeze/water cooling:
Antifreeze is just a water-equivalent way to transport heat - with boil point controlled by pressure (higher boil point at higher pressure). It is kept from flowing *through* the engine until warm by a (usually wax-actuated) valve. Other than looking at a flow map of the engine, that's about all there is to know.
 Sethersm
> ddavidn
Sethersm
> ddavidn
08/28/2013 at 15:03 |
|
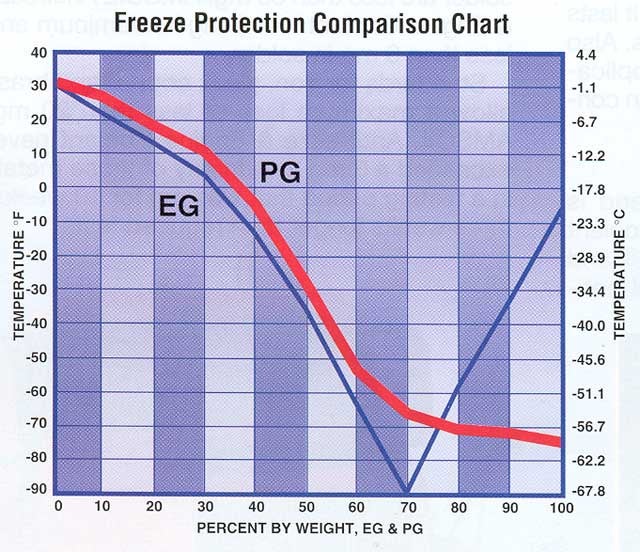
EG is Ethylene Glycol - Pure freezes and cracks your block at about -5ºF
PG is Propylene Glycol - Pure freezes and cracks you block at about -75ºF
 505Turbeaux
> ddavidn
505Turbeaux
> ddavidn
08/28/2013 at 15:05 |
|
Like feeding Gremlins after midnight...Never ever mix Land Rover O.A.T. with the green stuff. Results:


 Bandit
> ddavidn
Bandit
> ddavidn
08/28/2013 at 15:09 |
|
What about waterless coolant?
 HammerheadFistpunch
> ddavidn
HammerheadFistpunch
> ddavidn
08/28/2013 at 15:10 |
|
mixing coolant types (ethylene glycol/propylene glycol) is bad because the chemistry's aren't miscible so they will form a separation layer, like oil and water. Also, the additives will usually form a film that will clog the radiator (just replaced a radiator and coolant system due to this)
Antifreeze is basically just an additive to water the lowers the freezing point and raises the boiling point. Like adding salt to water, you can get hotter without changing mater phases or like adding alcohol lowers the freezing point. Since Antifreeze typically does more lowering of freezing point than raising of boiling point its best to use as little coolant as you can that will give you safe margins for the coldest your car will be operated. Due this within reason, I would never go more than 70% water. The water in the system is the actual coolant, its called antifreeze because...well it allows you to keep using your coolant (water) when it gets cold enough to freeze. That being said, don't ever add 100% antifreeze to a system, its hard to pump, doesn't do a very good job at being a coolant and will wreck your cooling system in a hurry.
Hope that helps
Side note: Ethylene glycol is toxic, Propylene glycol is generally considered less toxic/non-toxic and can in fact be added to foods like ice-cream safely.
 Sethersm
> Sethersm
Sethersm
> Sethersm
08/28/2013 at 15:12 |
|
I should also note that freeze protection is just one of the functions of antifreeze. In fact calling it antifreeze is only half the name, it's actually antifreeze/coolant. It also helps to raise the boiling point:
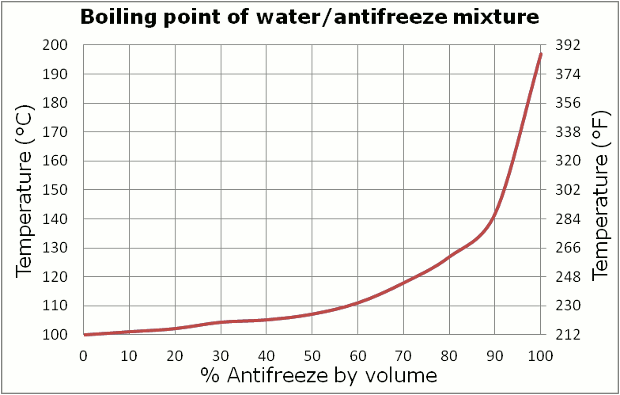
I'd guess that one is just ethylene glycol. I presume that doesn't take the pressure of the system into account, that will up the boiling point too.
Now, as to why mixing with water is desireable? Well, you could see with the first graph that with the ethylene glycol about 30% water gets you better freeze protection. Part of it might be cost? If you can get by with 50 antifreeze-50 water, going to 70 antifreeze-30 water is spending money on something you don't need?
 ddavidn
> HammerheadFistpunch
ddavidn
> HammerheadFistpunch
08/28/2013 at 15:13 |
|
Makes total sense. Ethylene is the green one, right?
 HammerheadFistpunch
> ddavidn
HammerheadFistpunch
> ddavidn
08/28/2013 at 15:14 |
|
I forget the color codes...I usually just make sure I'm getting the right chemistry. When it comes to my cars, I've found that sticking to the factory stuff or as close to factory specs as I can gives me the best cooling system life.
 ddavidn
> HammerheadFistpunch
ddavidn
> HammerheadFistpunch
08/28/2013 at 15:16 |
|
Subaru sells their own special coolant and special stop leak. However, I was at O'Reilly's which doesn't sell special Subaru coolant. I just know that the conventional green antifreeze was in there before, and I didn't want to mix types.
 RamblinRover Luxury-Yacht
> HammerheadFistpunch
RamblinRover Luxury-Yacht
> HammerheadFistpunch
08/28/2013 at 15:17 |
|
Worse, there are incompatible or non-ideal formulation differences between different PGs, IIRC. Also, some additives in the old days ate aluminum.
 HammerheadFistpunch
> ddavidn
HammerheadFistpunch
> ddavidn
08/28/2013 at 15:18 |
|
that's a safe rule of thumb. Subaru uses aluminum heat exchangers, so definitly stick to either PG or EG whichever they recommend (I forget which one is safe for AL, and which isn't)
 HammerheadFistpunch
> RamblinRover Luxury-Yacht
HammerheadFistpunch
> RamblinRover Luxury-Yacht
08/28/2013 at 15:20 |
|
Like I said, I just replaced the entire cooling system in my cruiser because the previous owner didn't stick to the cardinal rule of 80's series cooling systems: USE TOYOTA RED!
Running like a champ now.
 NaturallyAspirated
> ddavidn
NaturallyAspirated
> ddavidn
08/28/2013 at 15:23 |
|
Very simply put:
100% water is the most efficient coolant you can use in a car. It will transfer the most heat from the engine to the radiator.
The problem with 100% water is that it freezes at 32 degrees F, and since there are multiple types of metal inside most engines, it can promote corrosion.
So, you add antifreeze to the water in order to lower the freezing temperature and prevent corrosion.
Antifreeze, however, is much less efficient at cooling than water, so you don't want to use 100% antifreeze as your car would likely have trouble overheating when it's really hot outside.
The 50/50 mixture is an arbitrary "safe" mixture that is a good compromise of cooling efficiency and low freeze point and works well for most places in the world.
In Alaska it's common to run up to 30% water / 70% antifreeze for those -40 degree nights, while in the California desert it's not unheard of to run up to 70% water / 30% antifreeze.
 RamblinRover Luxury-Yacht
> HammerheadFistpunch
RamblinRover Luxury-Yacht
> HammerheadFistpunch
08/28/2013 at 15:31 |
|
Somehow, we have people who mix coolants that are f'ing RED AND GREEN, and blue brake fluid was still a problem. Meanwhile, any number of transmissions still out there will eat themselves without their proper boutique fill of Ford, Honda, or any other specialty brew - similar color notwithstanding.
Tell me again how attempting to legislate stupid away by barring certain colors in certain types of product works, when color differences would be more helpful *within* product types, and even then don't always stick. Nope, forget about stuff useful to people of average intelligence (red/green type distinction), let's make sure that one clown doesn't put brake fluid in his washer tank...
 ddavidn
> HammerheadFistpunch
ddavidn
> HammerheadFistpunch
08/28/2013 at 15:36 |
|
I'm pretty sure they recommend EG, which I'm pretty sure is green. Now I'm pretty sure I should double-check all that today...
 davedave1111
> ddavidn
davedave1111
> ddavidn
08/28/2013 at 16:18 |
|
The only reliable general advice is that it depends on what car/engine you're dealing with. Even if pure antifreeze would carry enough heat - it carries a bit less than water - it may rot the seals and so-on. There's no reason to do anything other than exactly what the manufacturer originally recommended, though - except maybe emergency fixes.
By the way, not enough people know this useful and amusing fact, but the antidote for ethylene glycol is... alcohol. If you accidentally get some antifreeze on your hands, you should have a beer just to be safe.
 davedave1111
> NaturallyAspirated
davedave1111
> NaturallyAspirated
08/28/2013 at 16:20 |
|
"100% water is the most efficient coolant you can use in a car."
Is it? I mean, the 'in a car' and working-temperature restrictions means we're not going to use liquid sodium or something, but I thought the reason for using water is that it's cheap and easy to work with. Oil as the working fluid will carry more heat, for example, but it's so much messier.
 ddavidn
> davedave1111
ddavidn
> davedave1111
08/28/2013 at 16:28 |
|
Do not fear, friend! I had plenty of bourbon while working on the car! I always play it safe.
 vdub_nut: scooter snob
> Bandit
vdub_nut: scooter snob
> Bandit
08/28/2013 at 21:17 |
|
Porsh pls....